RSV, or respiratory syncytial virus, typically causes cold symptoms and infects nearly everyone by age 2. But since last fall there has been an unprecedented spike in RSV that has contributed to a tripledemic, leaving families rightfully concerned, especially those with newborns and elderly adults, both of whom are at a higher risk of being hospitalized, according to the CDC. Right now there is currently no licensed RSV vaccine, but that could change relatively soon.
On March 1, a Food and Drug Administration advisory panel recommended approval of two vaccine candidates, one by Pfizer and the other by GSK, for adults ages 60 and older. Both still need formal approval by the larger agency, but the panel’s decision to recommend is a strong sign that the FDA will officially approve within a few months.
The CDC’s Advisory Committee on Immunization Practices met Feb. 23 to discuss the potential RSV vaccines, one for pregnant people and the other for the elderly, as well as the monoclonal antibody nirsevimab for infants. AstraZeneca and Sanofi are seeking FDA approval for monoclonal antibody treatments aimed at protecting newborns.
Most people recover from RSV infection within a week or two, but it’s particularly dangerous for newborns and the elderly. “For newborns, the danger is because of the immaturity of their immune system, which has not had exposure to this virus, as well as the low caliber of their airways, which can become clogged with mucus much faster,” Amesh A. Adalja, MD, senior scholar at the Johns Hopkins Center For Health Security, tells POPSUGAR. “For the elderly, it is the fact that they have many comorbid conditions that exacerbate the infection, as well as having immune senescence [or deterioration], with your immune system not responding appropriately as it once could.”
Health experts believe these candidates can alleviate the spike in RSV. “It’s likely that there will be multiple vaccines available, as well as nirsevimab, for next season, and together all should have a significant impact on the burden of illness caused by RSV in high-risk populations,” Dr. Adalja says.
Keep reading for a breakdown of the RSV vaccine candidates and what’s currently available to ward off infection.
Pfizer’s RSV Vaccine Candidates
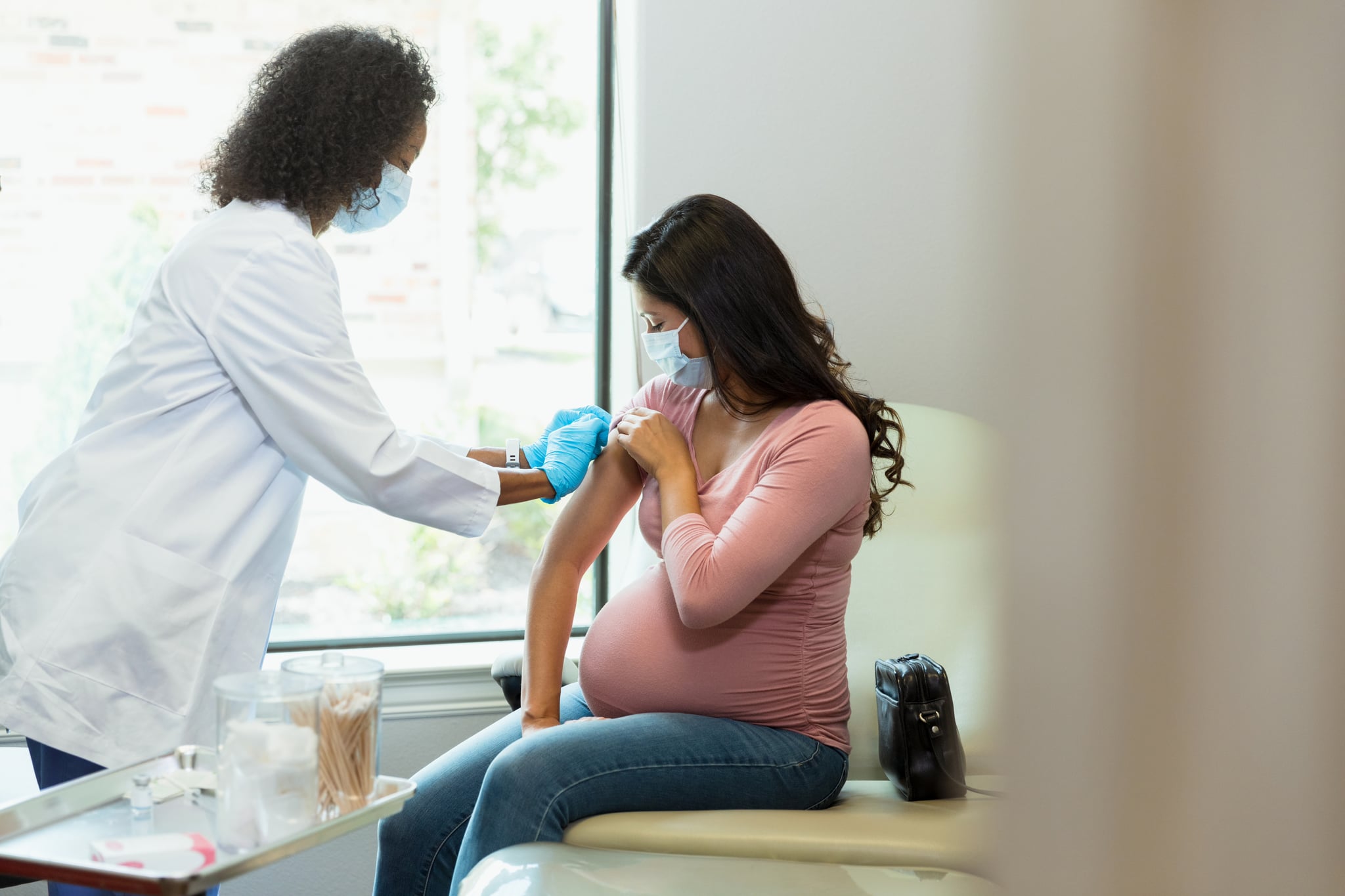
On Feb. 21, the FDA accepted Pfizer’s application for review of its vaccine, RSVpreF, which is being submitted for two separate approvals: for adults ages 60 and older and pregnant people to protect against RSV in newborns from birth through the first 6 months of life.
Pfizer Vaccine For Older Adults
Pfizer said in a statement that the vaccine has shown promising data in adults 65 and older. On March 1, a FDA advisory panel recommended approval for adults ages 60 and older. It still needs formal approval by the larger agency but the panel’s decision is a strong sign that the FDA will officially approve within a few months.
Prior to this recommendation, the FDA has asked Pfizer to conduct a safety study if the shot is approved now that two adults in their 60s who received Pfizer’s RSV vaccine were diagnosed with Guillain-Barre syndrome, per CNN. If you’re unfamiliar with the condition, Guillain-Barre syndrome is a rare neurological disorder where the body’s immune system attacks the nerves, according to Mayo Clinic. It may first present itself as weakness and tingling in the hands and feet, but can eventually lead to paralysis.
Pfizer Vaccine For Pregnant People
Pfizer is seeking separate approval for its RSV vaccine given to pregnant people in their last trimester. If approved, it would be the first vaccine administered to pregnant people to protect against RSV infection from birth through six months. But how does it work? “Certain antibodies are able to be transferred via placenta into the fetal circulation,” Dr. Adalja explains, essentially pregnant people can pass along antibodies they receive from the vaccine to their newborn. These antibodies would protect them from risk of RSV infection.
In Aug. Pfizer announced results from a Phase 3 clinical trial of more than 37,000 people. Its vaccine protected infants when given to pregnant participants between 24 and 36 weeks of gestation. The vaccine was more than 80% effective at preventing severe illness through the first 90 days after birth. At 6 months, it remained nearly 70% effective. On Feb 23, researchers said pregnant people who received the vaccine experienced moderate to mild side effects like redness, swelling and some pain at the injection site, per USA Today.
GSK Vaccine Candidate
GSK developed a candidate called called AReSVi, for older adults. Data published this month found the vaccine was more than 94% effective against severe RSV infection after nearly seven months. USA Today reported that the CDC panel debated on Feb 23 if the vaccine should be recommended for people 60 and older or 65 and older, but didn’t come to a conclusion. And on March 1, a FDA advisory panel recommended approval of the vaccine to the larger agency, which gives it a stronger chance of being formerly approved within the next few months.
Moderna’s Vaccine Candidate
On Jan. 17, Moderna announced that its RSV vaccine has shown to be 84 percent effective at preventing at least two or more symptoms in older adults (age 60 and older) and intends to submit a license application for regulatory approval in the first half of 2023. RSV infection causes more than 177,000 hospitalizations and 14,000 deaths among older adults every year, according to the CDC, so if approved would make a major difference in infection rates.
Sanofi’s Monoclonal Antibody
Sanofi and AstraZeneca have produced a single dose long-acting antibody called nirsevimab, which helps prevent RSV infection in infants entering their first RSV season. According to a clinical trial, nirsevimab reduced infections by 70% in healthy preterm infants born between 29 and 35 weeks. In January, the FDA agreed to review nirsevimab for approval. If approved, it would be the second monoclonal antibody on the market for infants.
Nirsevimab is not technically a vaccine, but it has been proposed by health experts that it should receive the same benefits of a vaccine such as being monitored by the Vaccine Adverse Event Reporting System (VAERS), in addition to making nirsevimab available through the Vaccines For Children program at no cost, and appearing on immunization records, per USA Today.
The CDC panel also noted that only one dose of nirsevimab is recommended per season but if given too early the effectiveness might wane before the risk of RSV has passed. Also due to COVID, interseasonal RSV transmission makes it trickier to determine when an infant should receive the single dose.
Synagis’s Monoclonal Antibody
Palivizumab, which goes under the brand name Synagis, is an FDA-approved prescription injection recommended for high-risk infants who were born severely premature at 29 weeks or earlier in order to protect them from severe RSV disease. Palivizumab is a monoclonal antibody — it’s not a vaccine, Dr. Adalja explains. Where a vaccine would train the immune system to fight off future infections, palivizumab is an immunizing agent which works by giving the body antibodies to protect it.